|
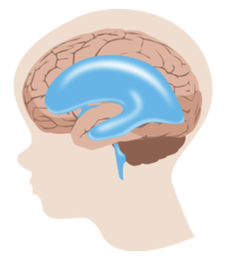
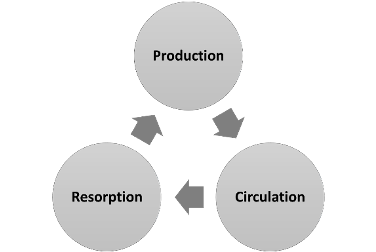
Everything you need to know about the function (and dysfunction) of cerebrospinal fluid Cerebrospinal fluid (CSF) physiology and hydrocephalus pathophysiology is a fundamental block of knowledge for all current and aspiring neurosurgeons. Emphasis on this topic is even more important for the pediatric neurosurgeon considering that manipulation of CSF makes up approximately 50% of all pediatric neurosurgical interventions. Cerebrospinal Fluid (CSF) Physiology CSF is a secretory fluid that circulates within the ventricular and subarachnoid (cranial and spinal) spaces serving both protective and metabolic functions for the central nervous system (CNS). CSF is a total volume of approximately 140 cc of clear, colorless, and largely acellular fluid that is produced at a constant rate. The largest volume of CSF exists in the subarachnoid space, while only about 25-40 cc are within the ventricles. CSF provides physical protection to the brain by creating a nearly neutral buoyancy that reduces the effective mass of the brain by roughly 95% (i.e. 1400-gram mass versus 25-gram effective mass). Reduced effective mass of the brain attenuates linear and rotational accelerations that would otherwise result in damaging impulses against the inner calvarium. Additionally, CSF continually “washes” the brain via dilution and clearance of the byproducts of metabolism and neurotransmission. Thus, a vital physiologic function is the ability of central chemoreceptors to sense inappropriate buildup of such metabolites in the CSF. It is helpful to conceptualize CSF physiology in three main compartments (Figure 1): production, circulation, and resorption. CSF is an ultrafiltrate of blood plasma. Due to the rigid nature of the skull, it is a general rule of thumb that CSF produced must be balanced by an equivalent rate of resorption. Disruption of this finely balanced physiology can lead to pathology. Figure 1: Equal amounts of CSF must be produced and resorbed at a constant rate to maintain homeostasis within the CNS. CSF Production Production of CSF results from specialized filtration of plasma through the blood brain barrier (BBB) at discrete locations in the brain: choroid plexus (90% of production), parenchyma (10-20%), and ependyma (< 1%). Specifically, the interface between the capillaries and the epithelium lining those anatomical niches are responsible for CSF production. Thus, a comprehensive discussion of CSF physiology should include reviewing the blood brain barrier (BBB). The purpose of the BBB is to ensure that the CNS remains an immune-privileged environment. For substrate to pass from the capillary lumen to the intraventricular/cisternal space, it must traverse the following:
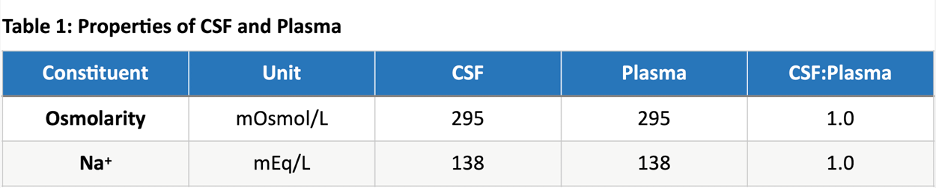
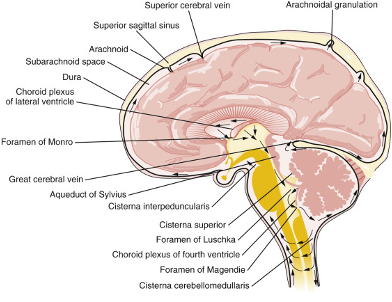
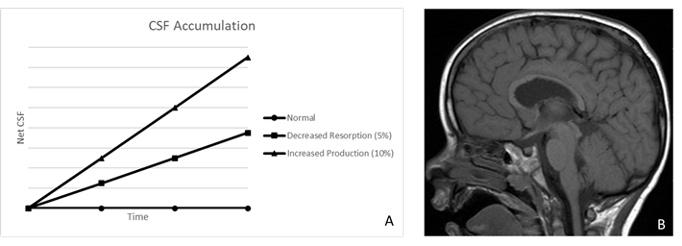
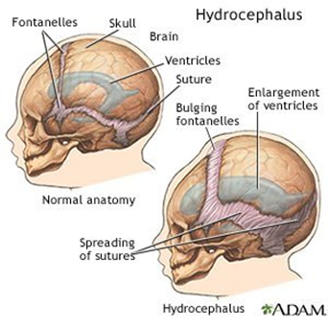
Variation in BBB restriction throughout the brain yields unique plasma filtration properties in appropriate anatomical locations, such as the production of CSF as an ultrafiltrate of plasma in the choroid plexus. Additionally, of note, there are several non-privileged areas of the brain surrounding the third and fourth ventricles where the BBB is nonexistent and substrate diffusion is rapid: Vascular organ of the lamina terminalis, subforniceal organ, median eminence, adenohypophysis, subcommissural organ, pineal gland, and area postrema. The majority of CSF is produced via filtration of blood plasma in the choroid plexus – a villous epithelium comprised of a single cell layer of simple cuboidal ependymal cells joined by tight junctions. The capillary bed abutting the high surface volume (>200 cm2) villous epithelium of the choroid plexus consists of fenestrated endothelium, with relatively little inhibition of plasma filtration on a macroscopic level. Ependymal cell production of bicarbonate generates a proton gradient that powers influx of Na+ across the basal (capillary) surface. Na+/K+ ATPase drives sodium efflux against a chemical gradient across the apical (ventricular) cell membrane, yielding a net-negative intracellular membrane charge that favors efflux of negatively charged bicarbonate into the ventricular space. Free water follows the osmolar gradient from the intracellular space to the intraventricular compartment. CSF is produced with an approximate osmolarity of 295 mOsmol/L and a Na+ concentration of approximately 138 mEq/L., Of note, the ratios of CSF to Plasma osmolarity and Na+ concentrations should both be close to 1 (Table 1). CSF is produced at a constant rate between 0.35-0.4 cc/min (20-24 cc/hr, 500-600 cc/day), leading to complete volume replacement 4-5 times/day. Homeostasis is dependent on balance between CSF production and resorption. Pathologic increases in CSF production may be due to choroid plexus tumors, choroid plexus hypertrophy, cholera toxin, or sympathectomy procedures leading to modified autonomic innervation of the choroid plexus. Alternatively, decreased CSF production occurs in the settings of reduced cerebral blood flow (CBF), infection, hypothermia, and hypoglycemia. Of note, intracranial pressure (ICP), osmolality, and age do not affect CSF production. CSF Circulation The majority of CSF production occurs in the lateral ventricles. Via pulsatile waves, CSF flows from the lateral ventricles through the foramina of Monro into the third ventricle, through the aqueduct of Sylvius into the fourth ventricle, and through the foramina of Magendie and Luschka into the subarachnoid/cisternal spaces where it is eventually resorbed into the venous system through arachnoid granulations (Figure 4). The ventricular spaces comprise approximately 24-40 cc of volume, compared to 90-110 ccs of the subarachnoid spaces. CSF Resorption The majority of CSF is resorbed via the transventricular pathway; meaning, after production in the lateral ventricles, CSF traverses the lateral-to-third-to-fourth ventricles into the subarachnoid space where it is resorbed into the venous blood through arachnoid granulations (Figure 2). Arachnoid granulations are outpouchings of the arachnoid membrane (villi) abutting venous sinuses that increase surface area for unidirectional resorption of CSF – the largest collection being along the superior sagittal sinus. A pressure gradient mediates resorption between the subarachnoid space and venous sinus (∆P = Psubarachnoid space - Pvenous sinus). A much smaller portion of CSF is resorbed through a poorly characterized glymphatic system. When CSF resorption compensation is needed (e.g. in the setting of a relative overproduction of CSF), clinical evidence of leptomeningeal, perivascular, and/or perineural lymphatic engorgement may be seen. Manipulating the pressure gradient between the subarachnoid space and venous sinus can positively or negatively influence the rate of CSF resorption. Decreasing intravenous pressure or increasing subarachnoid pressure will increase the rate of CSF resorption. Decreased intravenous pressure may be accomplished by raising the head of a patient’s bed, decreasing vascular oncotic pressure, or decreasing vascular volume. Alternatively, increased intravenous pressure (i.e. neck pressure/restrictive swelling) or decreased subarachnoid pressure will result in decreased CSF resorption. Figure 2: Cerebrospinal fluid (CSF) pathway. The diagram depicts the structures and spaces through which CSF flows, from its production in and secretion from the choroid plexus in the ventricles, to its ultimate resorption from the subarachnoid space. CSF exits the lateral ventricles through the foramina of Monro into the third ventricle. It then traverses the aqueduct of Sylvius into the fourth ventricle, leaves through the foramina of Lushka andMagendie,passes into the cisterna magna, and flows through the subarachnoid space around the cerebral hemispheres and spinal cord. Hydrocephalus Pathophysiology Hydrocephalus is a pathologic state of excess CSF due to an imbalance between production and resorption. Hydrocephalus occurs in 1/2000 births and results from a variety of either primary or secondary etiologies. In the pediatric population, approximately 50% of all neurosurgical procedures (estimate 33,000 annual procedures) are directed towards restoring balance in CSF physiology, with healthcare costs well beyond $94 million. Etiologies of hydrocephalus can be considered in three main categories (Figure 3): increased production, impaired circulation, and blocked resorption. Figure 3: A) Net accumulation of CSF can result from increased production or decreased resorption. Obstruction to flow leads to proximal CSF build-up, as seen in B. B) Preoperative MRI with apparent aqueductal obliteration. Communicating hydrocephalus occurs due to pathologies that increase CSF production (non-obstructive hydrocephalus) or block CSF resorption (extraventricular-obstructive hydrocephalus; EVOH). Choroid plexus tumors are the most common pathology resulting in increased CSF production. Decreased absorption is typically due to pathologic states that disrupt the subarachnoid-venous interface such as subarachnoid hemorrhage (clot obstruction), meningitis (inflammatory obstruction), or venous hypertension (reversed pressure gradient obstruction). Both forms of communicating hydrocephalus result in a global increase in CSF volume. Non-communicating hydrocephalus results from a distinct obstruction that impairs CSF circulation yielding increased volumes upstream from the obstruction and decreased volumes downstream. Non-communicating hydrocephalus may also be referred to as intraventricular-obstructive hydrocephalus (IVOH). Intraventricular obstruction may result from tumors (intraventricular mass or extraventricular mass compressing ventricular outlets), anatomic malformation, ventriculitis (blockage by inflammatory debris), or colloid cysts (benign, congenital mass commonly found on the floor of the third ventricle). Hydrocephalus can be a life-threatening pathology that requires urgent intervention to maintain intracranial pressure (ICP). Thus, comprehensive discussion of hydrocephalus necessitates review of the Monro-Kellie Doctrine and ICP dynamics. Due to the fixed volume of the calvarium, increased volume of brain (i.e. swelling), blood (increase cerebral blood flow or decreased venous drainage), or CSF (i.e. hydrocephalus) necessitates a compensatory decrease in volume of one or more other mediums in order to prevent an increase in ICP). Ideally, ICP should be maintained between 7-15 mmHg and cerebral perfusion pressure (CPP) >60 mmHg. Compensatory mechanisms can maintain normal ICP for up to approximately 100-120 mL of increased CSF volume. ICP will rapidly increase as CSF volume increases beyond this threshold and compensatory mechanisms are exhausted. In young, pediatric patients, viscoelastic properties of the immature cranium and open fontanels/cranial sutures allow for significant cranial expansion to accommodate elevated CSF/ventricular volumes (Figure 4). Figure 4: Cranial suture and fontanel morphology in an infant with and without hydrocephalus. Clinical Assessment of Hydrocephalus Increased CSF volume and elevated ICP in older children and adults may present with headache, morning emesis, cognitive impairment, ataxic gait, and/or urinary incontinence (mnemonic for communicating hydrocephalus: “wet, wild, and whacky”). Infants and young children may present with macrocrania (divergent head circumference due to cranial volume expansion), feeding intolerance, irritability, delayed development, upward gaze paresis (“setting sun” gaze), cranial suture diastasis (suture separation or splaying), full/tense fontanelles, and/or engorgement of scalp veins (Figure 5). Figure 5: Morphologic features exhibited in hydrocephalus. MRI is the gold standard of diagnosis for hydrocephalus. It provides a high-resolution view of anatomical features, variable sequences for treatment planning and volumetric assessment, and motion-sensitive sequences for evaluation of CSF flow dynamics. Standardized 2D imaging measurements are commonly used in diagnosis (Figure 6). 2D phase contrast MRI (CINE MRI) provides a greyscale image of motion. This type of imaging is useful for determining the direction and velocity of flow through specific anatomic landmarks such as the aqueduct of Sylvius, cisterna magna, or post-operative flow through the stoma (opening) of a third ventriculostomy. Lastly, fetal MRI is being increasingly utilized for prognostication and treatment planning of unborn children. Figure 6: Postoperative MRI 2D phase contrast with third ventricle CSF flow evidence.
Treatment of Hydrocephalus Pharmacological Hydrocephalus is rarely due to an increase in CSF production, but rather more commonly, the result of interrupted circulation or decreased absorption. Just as very few pathologies influence the rate of CSF production, it is also difficult to pharmacologically manipulate as this system is tightly regulated. In general, CSF production is relatively constant and difficult to modulate. However, there are pharmacologic agents capable of modestly temporizing CSF production for brief periods of time. Acetazolamide (Diamox) is a carbonic anhydrase inhibitor that reduces conversion of carbon dioxide to bicarbonate in ependymal cells (among other targets), resulting in dissipation of the proton gradient that would otherwise drive the influx of Na+ (with free water) across the basal (capillary) surface of ependymal cells. Acetazolamide also acts on the proximal convoluted tubule of the kidneys, resulting in increased excretion of bicarbonate, Na+, Cl-, and water that can lead to metabolic acidosis and compensatory hyperventilation. Cardiac glycosides (Oubain, Digitoxin) inhibit Na+/K+ ATPase on the apical (ventricular) membrane of ependymal cells. Reduced efflux of Na+ (and free water) into the ventricular space results in loss of the Na+ gradient that drives net migration across ependymal cells. Lastly, atrial natriuretic peptide (ANP) acts on surface receptors of ependymal cells to reduce CSF production via cGMP-mediated secondary messenger modulation of aquaporin channel expression. Aquaporin 1 (AQP1) channels directly control permeability of free water; blockade or downregulation of expression will lead to decreased CSF production. Situations which warrant use of carbonic anhydrase inhibitors include temporizing maneuvers such as waiting for surgery, patient transport, or waiting for a reversible process to take hold. While these drugs are useful in the short-term, long-term usage can be problematic due to the eventual onset of metabolic acidosis. Surgical Most hydrocephalus intervention is performed surgically. Temporary treatments or techniques available while waiting for resolution include lumbar puncture, ventricular tap, extraventricular drainage, and ventriculosubgaleal shunts. More definitive treatments may be required in the setting of obstructive (non-communicating) hydrocephalus, in which removal of the obstructive lesion is the best course of action as removal allows for the re-establishment of natural CSF pathways. This also prevents the need for permanent shunting. Other treatment options include elimination of the source of CSF production (i.e. choroid plexus fulguration or coagulation), creation of an artificial conduit (i.e. diversion procedure), shunt insertion, aqueductoplasty/foraminoplasty, and third ventriculostomy. Shunt placements account for about 50% of all pediatric neurosurgical procedures as they are extremely durable, preserve patient life and well-being, and allow for decreased ventricle size while the parenchymal compartment expands. Aqueductoplasty/foraminoplasty re-establish normal CSF flow, and third ventriculostomies re-establish flow from the intraventricular compartment to the subarachnoid space by opening up the bottom of the third ventricle to bypass the site of obstruction. The latter procedures are most effective in treating non-communicating hydrocephalus and aid in eliminating the shunt burden. References: Brichtova E, Chlachula M, Hrbac T, Lipina R. Endoscopic third ventriculostomy in previously shunted children. Minim Invasive Surg. 2013;2013:584567. Cochard, Larry R. Netter's Atlas of Human Embryology. Philadelphia: Elsevier, 2012. Web. Guyton and Hall Textbook of Medical Physiology - 14th Edition Hydrocephalus in Children (Aftercare Instructions) - What You Need to Know Pediatric Hydrocephalus Seminar 1: Cerebrospinal Fluid Physiology & Hydrocephalus Pathophysiology - YouTube Swaiman's Pediatric Neurology. Abou-Hamden, Amal; Drake, James M... Published January 1, 2017. Pages e561-e576. © 2017. Authors: Ryan Radwanski, Gretchen Koller, Monica Mureb, Anthony Anzalone, Alexander Kelly Comments are closed.
|
Categories
All
Archives
October 2023
|









2/9/2021